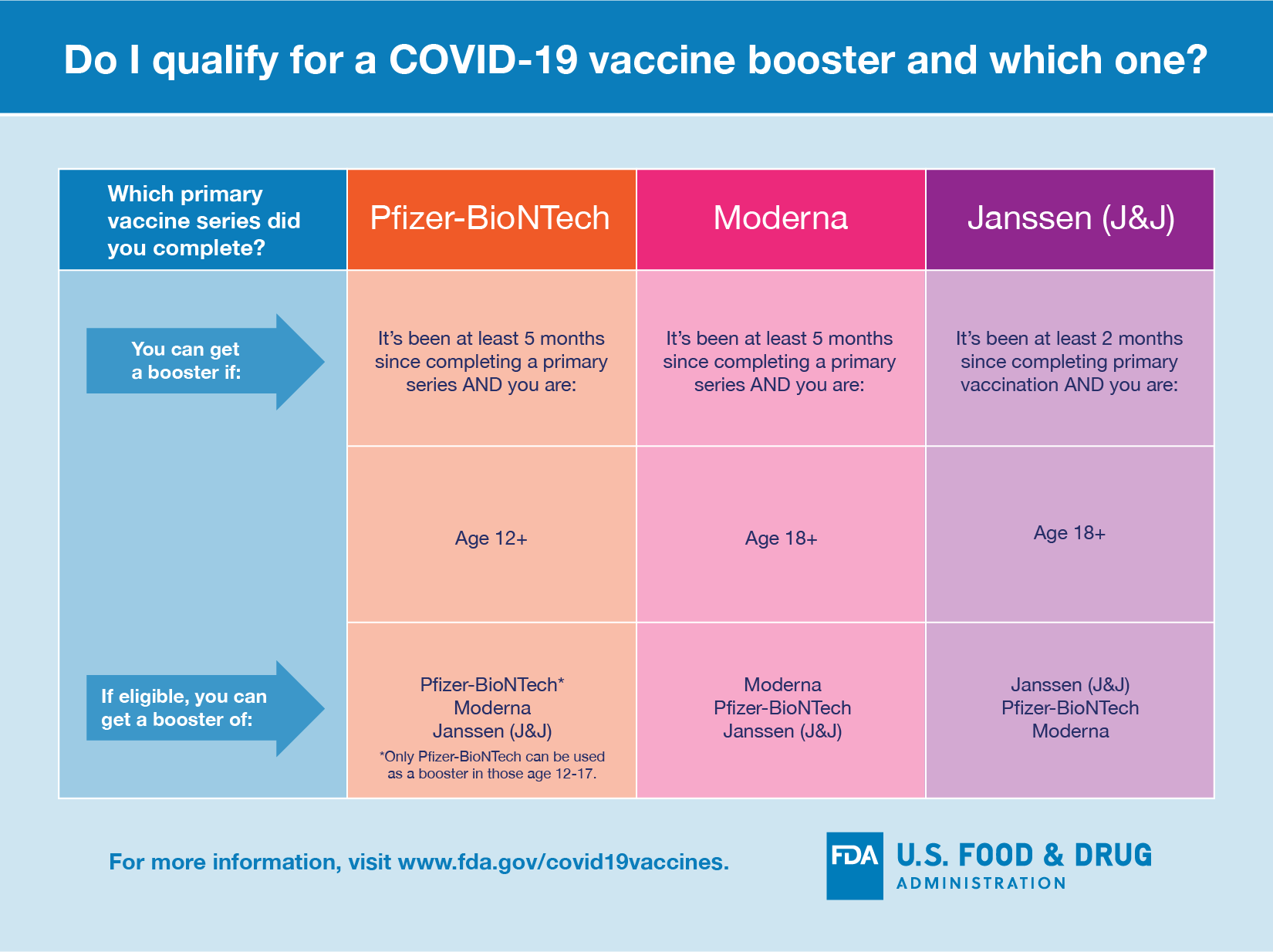
Coronavirus (COVID-19) Update: FDA Shortens Interval for Booster Dose of Moderna COVID-19 Vaccine to Five Months | FDA
FDA weighs booster shot recommendations as COVID-19 cases see slight dip – Zilber College of Public Health

FDA Panel Says Pfizer COVID Booster OK For Older People And Those At High Risk : Coronavirus Updates : NPR

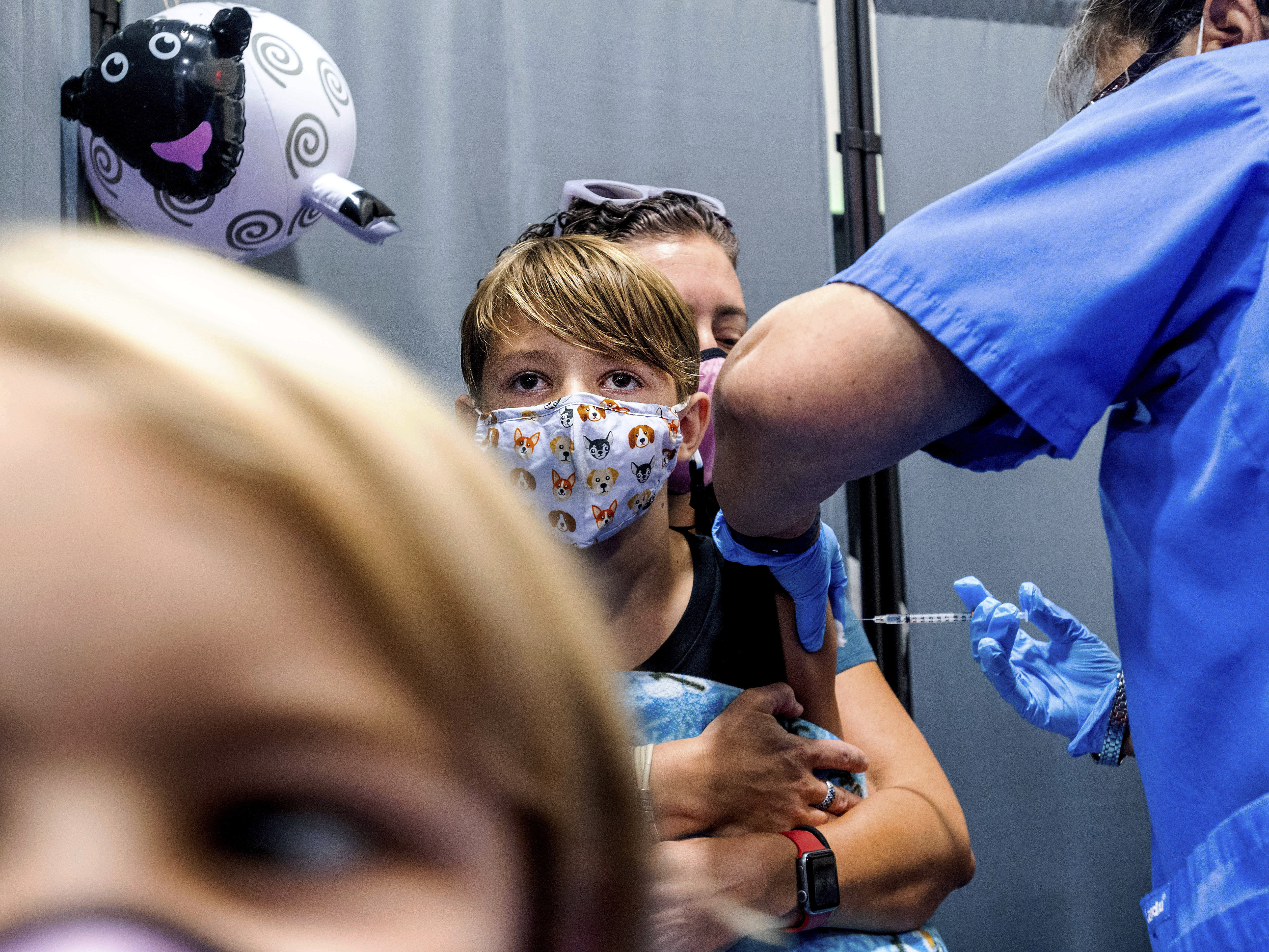
CDC recommends new COVID booster for all Americans over 6 months amid rising cases, hospitalizations - ABC News
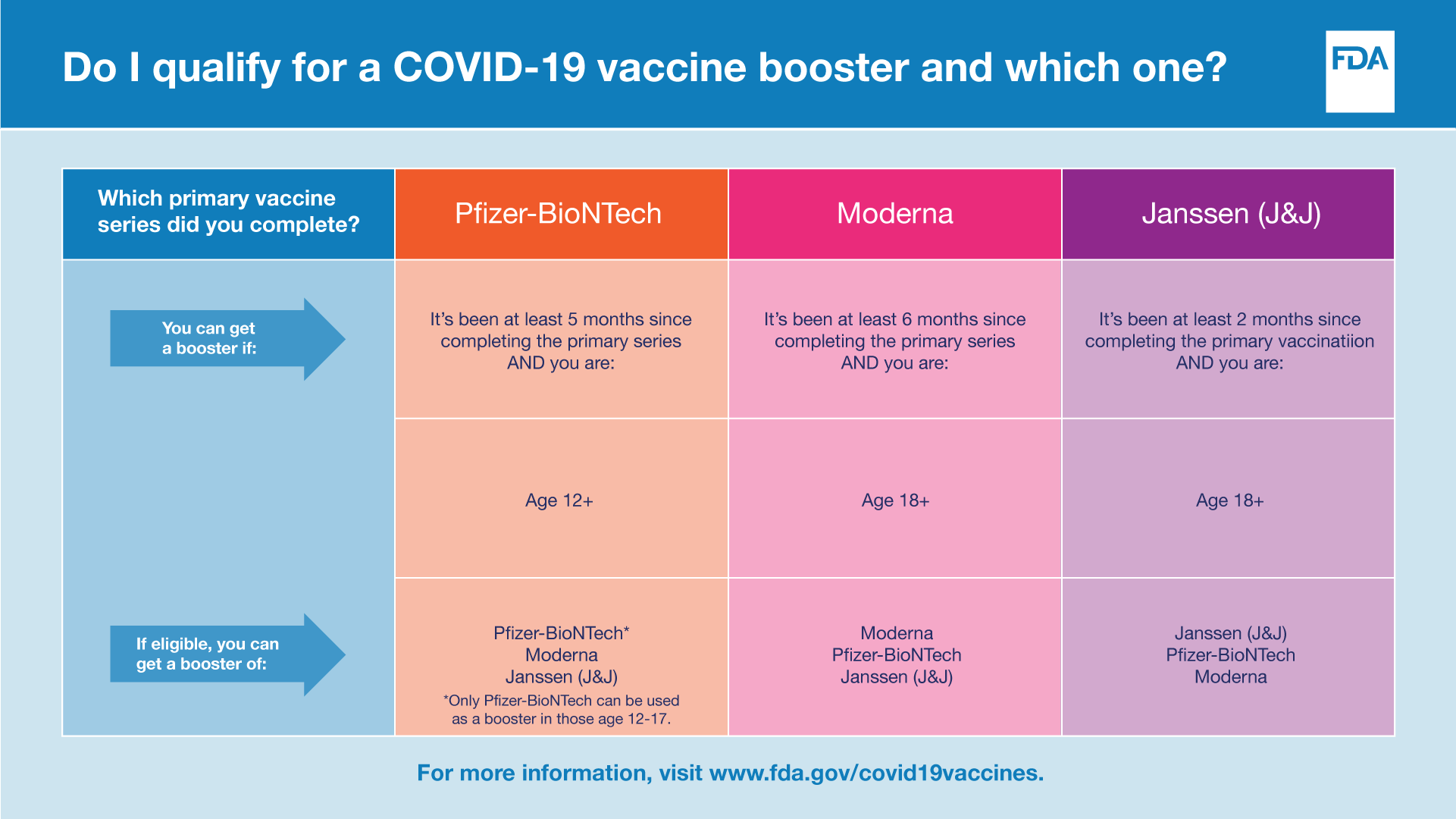
Coronavirus (COVID-19) Update: FDA Takes Multiple Actions to Expand Use of Pfizer-BioNTech COVID-19 Vaccine | FDA

FDA committee meets to debate and vote on Covid booster shots for the general public — 9/17/21 - YouTube
FDA Advisers Say Next Round of COVID Booster Shots Should Target an XBB Variant - Southern Iowa Mental Health Center

U.S. FDA on X: "Today, we amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 Vaccine to allow for use of a single booster dose, to be administered at least 6
FDA greenlights Pfizer booster shot for certain groups; CDC advisory panel votes to recommend boosters | AHA News