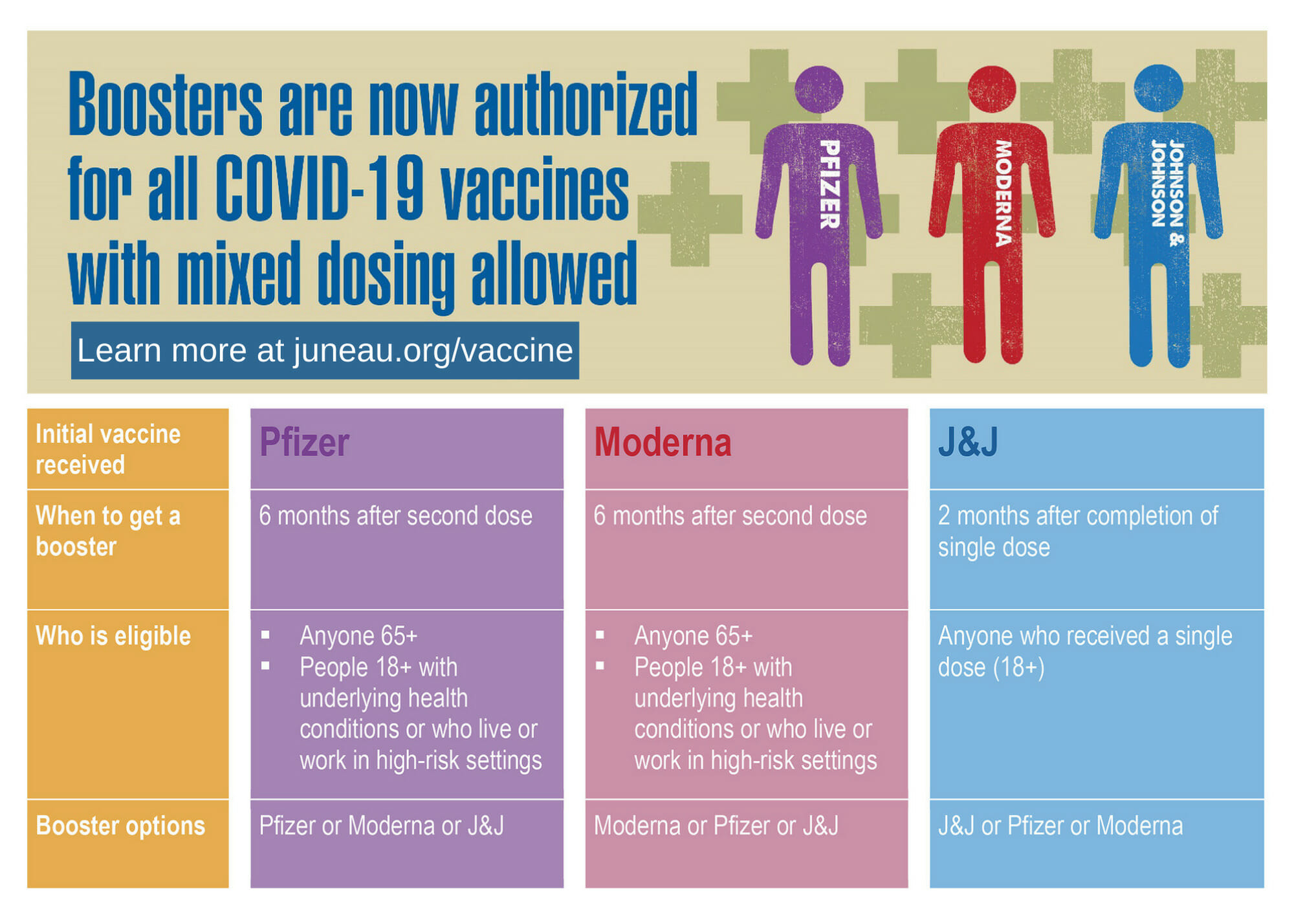
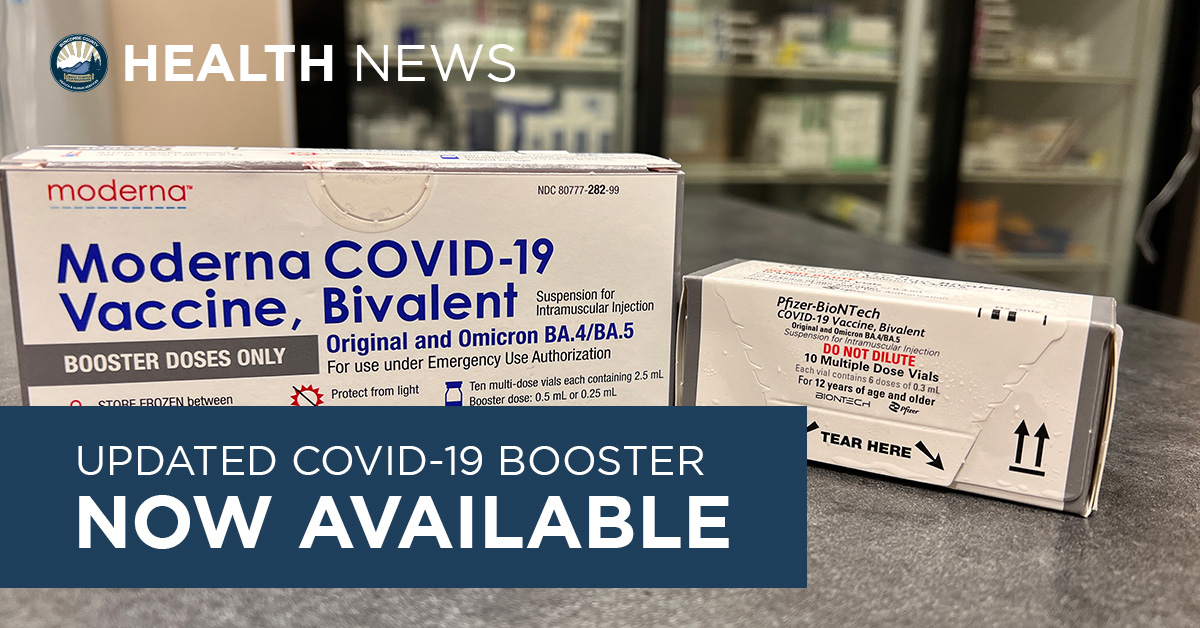
OCHD ANNOUNCES MODERNA AND J&J BOOSTER AVAILABLE AT VACCINATION CLINICS FOR ELIGIBLE RESIDENTS – Ocean County Health Department
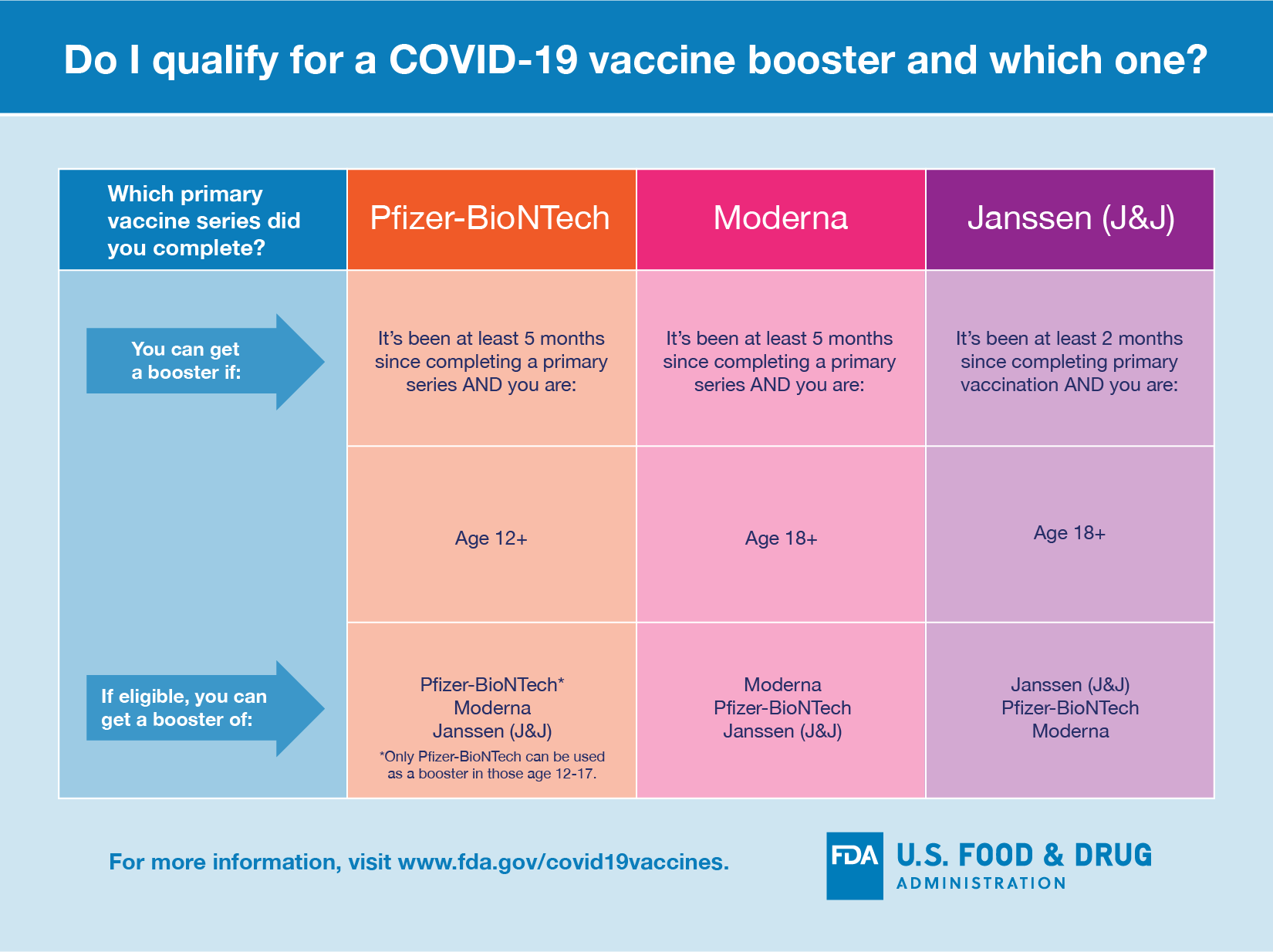
Coronavirus (COVID-19) Update: FDA Shortens Interval for Booster Dose of Moderna COVID-19 Vaccine to Five Months | FDA
CDC recommends additional booster of Pfizer-BioNTech, Moderna Covid-19 vaccine for adults 50 and older - POLITICO

With new Moderna booster in short supply, get whatever shot's available, doctors say | Ideastream Public Media


/cdn.vox-cdn.com/uploads/chorus_asset/file/23006448/GettyImages_1236355935.jpg)